Monday 15 October
Shells and the hard body parts of corals and crustaceans rely on a mineral called calcium carbonate for their structure. But calcium carbonate (CaCO3) takes many forms, and a rare form was recently discovered in sea ice.
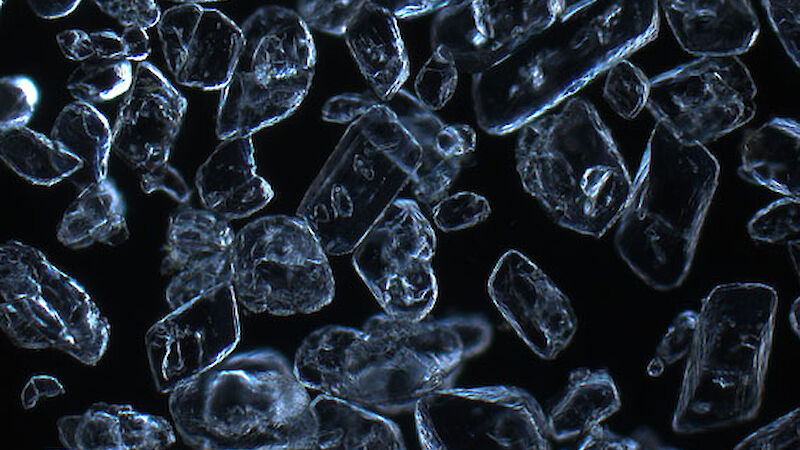
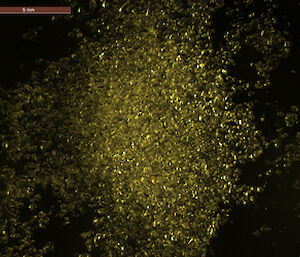
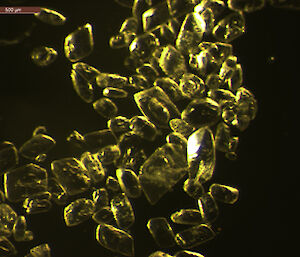
Dr Gerhard Dieckmann from the Alfred Wegener Institute for Polar and Marine Research in Germany was among those who obtained the first evidence of rare calcium carbonate crystals, known as ikaite, in Antarctic sea ice in 2001. Now Dr Dieckmann is part of the SIPEX II voyage, searching for these ‘diamonds in the sea ice’.
“These crystals appear when sea ice forms,” Dr Dieckmann says.
“The very salty brine in the sea ice undergoes physical and chemical changes that lead to the precipitation of ikaite from the briny liquid. But the distribution of these crystals in sea ice is patchy.
The significance of these crystals in sea ice is not fully understood, but scientists think they may play a role in the sea ice-driven carbon pump (the movement of carbon from the sea ice to the ocean below), through the release of carbon dioxide when the crystals form. Ikaite formation may also cause acidification of sea ice brine, triggering the release of ozone-destructive bromine from sea salt. A better understanding of the role of ikaite in the carbon cycle and its distribution in sea ice will therefore allow scientists to improve climate and ecosystem models.
To determine the quantity and distribution of ikaite in sea ice Dr Dieckmann takes ice cores back to the lab and melts 10cm segments at 4°C — any warmer than this and the crystals disappear. Once melted, the crystals appear like a sprinkling of salt in the bottom of a container. Dr Dieckmann measures their concentration by weighing dried crystals. But it’s under the microscope that their beauty becomes apparent; they truly are sea ice diamonds.